ShopDreamUp AI ArtDreamUp
Deviation Actions
Description
Digital doodle using Adobe PhotoShop
Image size
355x410px 115.9 KB
© 2015 - 2024 Calilasseia
Comments1
Join the community to add your comment. Already a deviant? Log In
For those wondering about this latest addition to my artworks, it was inspired by thoughts arising from reading parts of Derek Lowe's blog. Derek Lowe is a chemist, and one who possesses a true gift for explaining all manner of arcane chemical reactions (and their products) to even a non-technical audience - think of his Things I Won't Work With articles as the sort of writing Carl Sagan might have produced if he wrote about scary chemistry, and you won't go far wrong. 
In particular, Derek Lowe has a special soft spot for introducing people to the wonders going on inside the Klapötke lab in Munich, a facility whose researchers are devoting their paid employment time to synthesising all manner of hideously explosive new compounds, containing large numbers of nitrogen atoms bonded together, using bonding structures that those nitrogen atoms would, if they were sentient, very much prefer NOT to be bonded by. There are a whole range of these, including azides (many of which have been well-known primary explosives for decades), but the Klapötke lab is exploring a part of the chemical landscape that has "Here Be Dragons" hanging over it in very large letters.
So, having seen a recent outing by these chemists, documented by Derek Lowe in his inimitable style here, and having spent enough time learning some chemistry in the past to entertain some interesting speculative thinking, motivated by his blog, I thought I'd put this together.
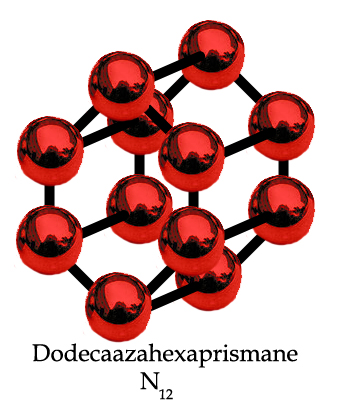
Now, I have NO idea if it's possible to synthesise this compound, which, if it ever IS synthesised, will probably be not only very powerfully explosive, but possibly even more unstable than the latest batch of aziodoazide azides described on that blog page. But, if it ever IS possible to synthesise the above molecule, and it transpires to be stable enough to extract some structural data from without exploding (presumably at temperatures in the 50 Kelvin range ... ?), it will be an interesting scientific first for the chemists who succeed, because, that molecule above is a theoretically possible allotrope of nitrogen. The emphasis being very strongly on the word "theoretical", unless of course some research chemists out there have already worked out a synthesis and are due to publish their results in JACS or Angewandte Chemie. If chemists ever do find a way of synthesising that molecule, it may possess some interesting properties.
Anyway, it also gave me an opportunity to come up with a nice name for it. Since it has 12 nitrogen atoms bonded together, in two hexagonal rings of 6 atoms further joined together by other bonds to form a hexagonal prism shape, what better name than "dodecaazahexaprismane"?
In particular, Derek Lowe has a special soft spot for introducing people to the wonders going on inside the Klapötke lab in Munich, a facility whose researchers are devoting their paid employment time to synthesising all manner of hideously explosive new compounds, containing large numbers of nitrogen atoms bonded together, using bonding structures that those nitrogen atoms would, if they were sentient, very much prefer NOT to be bonded by. There are a whole range of these, including azides (many of which have been well-known primary explosives for decades), but the Klapötke lab is exploring a part of the chemical landscape that has "Here Be Dragons" hanging over it in very large letters.
So, having seen a recent outing by these chemists, documented by Derek Lowe in his inimitable style here, and having spent enough time learning some chemistry in the past to entertain some interesting speculative thinking, motivated by his blog, I thought I'd put this together.
Now, I have NO idea if it's possible to synthesise this compound, which, if it ever IS synthesised, will probably be not only very powerfully explosive, but possibly even more unstable than the latest batch of aziodoazide azides described on that blog page. But, if it ever IS possible to synthesise the above molecule, and it transpires to be stable enough to extract some structural data from without exploding (presumably at temperatures in the 50 Kelvin range ... ?), it will be an interesting scientific first for the chemists who succeed, because, that molecule above is a theoretically possible allotrope of nitrogen. The emphasis being very strongly on the word "theoretical", unless of course some research chemists out there have already worked out a synthesis and are due to publish their results in JACS or Angewandte Chemie. If chemists ever do find a way of synthesising that molecule, it may possess some interesting properties.
Anyway, it also gave me an opportunity to come up with a nice name for it. Since it has 12 nitrogen atoms bonded together, in two hexagonal rings of 6 atoms further joined together by other bonds to form a hexagonal prism shape, what better name than "dodecaazahexaprismane"?